Date: 12 March 2019 By Dr Heidi Gardner #trialretention
 Randomised clinical trials are the gold standard method for gathering evidence about health and care interventions. That said, randomised trials are not perfect, and many of them run into issues through the process of trial design and delivery.
Randomised clinical trials are the gold standard method for gathering evidence about health and care interventions. That said, randomised trials are not perfect, and many of them run into issues through the process of trial design and delivery.
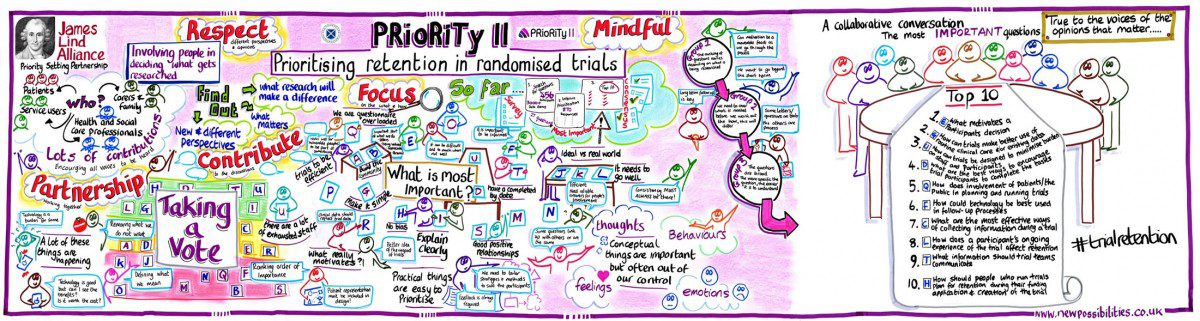
Participants are central to the successful completion of trials; without trial participants researchers would be unable to collect the data needed to answer the question that is central to the trial’s existence. Naturally, that has led to researchers spending significant time and effort on attempting to solve the problem of poor recruitment – the thought process here being that if we can encourage more people to take part in trials, then the likelihood of collecting sufficient data is increased. Unfortunately, once participants are enrolled in a trial, they are not always retained until the trial is complete. Research into reasons behind poor participant retention in trials is limited, and the research community is struggling to get to grips with methods to improve retention as a result. CLICK HERE TO READ THE FULL ARTICLE.